What is the source of our energy?
Energy in the human body is derived from the three macronutrients present in food, namely carbohydrates, proteins, and lipids. During the digestive process, these complex molecules are broken down into smaller molecules for absorption. The body uses enzymes to break down proteins into amino acids, polysaccharides (complex sugars) into simple sugars, and lipids (fats) into fatty acids and glycerol. (3)
How is energy made in the body?
Our cells use glucose, a simple sugar, to produce energy via a process known as aerobic cellular respiration. Human energy production relies on mitochondria, referred to as the body’s “powerhouses”, which are organelles found within the body’s cells. Mitochondria are involved in intracellular signaling and the metabolism of amino acids, carbohydrates, lipids, and steroids. (19)
Cellular respiration begins when glucose enters the cytosol of the cell and involves a series of reactions, including:
- Glycolysis: glucose is converted to pyruvate, which then passes into the mitochondria where it is broken down into CO2 and acetyl-CoA is formed. A small amount of the energy carriers, adenosine triphosphate (ATP) and nicotinamide adenine dinucleotide hydrogen (NADH), are produced in this step.
- The Krebs cycle (or citric acid cycle): acetyl-CoA then passes into the mitochondrial matrix, where the acetyl group is oxidized and a large amount of NADH is produced.
- Oxidative phosphorylation (or electron transport chain & chemiosmosis): NADH from the Krebs cycle moves to the inner mitochondrial membrane, where energy is released through the transfer of electrons from NADH is used to produce a large amount of ATP. (1)
The ATP produced in the mitochondria allows for the redistribution of energy used throughout the cell, supporting its specific functions. (1) The complex set of reactions outlined above requires the input of various nutrients used as cofactors in the reactions, including:
- Coenzyme Q10 (CoQ10)
- Thiamine (vitamin B1)
- Riboflavin (vitamin B2)
- Niacin (vitamin B3)
- Pantothenic acids (vitamin B5)
- Magnesium (8)
Low energy and fatigue
In the United States, approximately one in five individuals seeking care from their family physician report symptoms of fatigue. (14) Fatigue is characterized by overwhelming feelings of tiredness that are not resolved by restful sleep. (9) Research suggests that a cause cannot be identified in approximately one third of cases of chronic fatigue. (14)
Several causes of low energy levels have been suggested, many of which are correlated to mitochondrial dysfunction. Certain lifestyle factors may impair the integrity and function of your mitochondria, such as antibiotics and other medications, chronic stress, environmental toxins, hyperglycemia (elevated blood sugar), and sleep disturbances. (8)
Mitochondrial dysfunction may be characterized in several ways, including:
- Insufficient number of mitochondria
- Insufficient cofactors required for cellular respiration (e.g., nutrient deficiency due to poor diet or certain medications)
- Dysfunction in the synthesis of ATP
- Damaged mitochondrial membranes (8)
When mitochondrial damage or dysfunction occurs, the result is a decreased production of ATP and an increased production of reactive oxygen species (ROS). An overabundance of ROS increases oxidative stress in the body, which is associated with certain conditions including fatigue, aging, atherosclerosis, cancer, diabetes, and neurodegeneration. (8)
Additionally, chronic inflammation may contribute to or aggravate fatigue. Researchers have identified higher levels of pro-inflammatory compounds in individuals with fatigue, including C-reactive protein (CRP), tumor necrosis factor alpha (TNF-α), and interleukin-6 (IL-6). (9)
How to support energy levels naturally
Energy support may involve reducing inflammation and improving antioxidant status in the body. (9) Lifestyle modifications include incorporating healthy foods that give you energy, dietary supplements, regular physical activity, and minimizing exposure to environmental toxins.

Diet
Several dietary patterns may benefit individuals with fatigue, including the anti-inflammatory diet, the Mediterranean diet and the leaky gut diet. One study in breast cancer survivors found that non-fatigued survivors consumed diets higher in antioxidant and anti-inflammatory properties than fatigued survivors. This led to the development of the fatigue reduction diet (FRD), an eating pattern high in fish, whole grains, and fruits and vegetables, particularly leafy green vegetables and tomatoes. (9) A subsequent controlled trial examined the effects of following the FRD over three months, which was associated with decreased blood levels of saturated fatty acids, as well as increased levels of carotenoids and omega-3s in the diet group. Improvements in fatigue and sleep quality were also seen in the FRD group. (18) The following table summarizes foods to enjoy and limit to help improve your energy levels.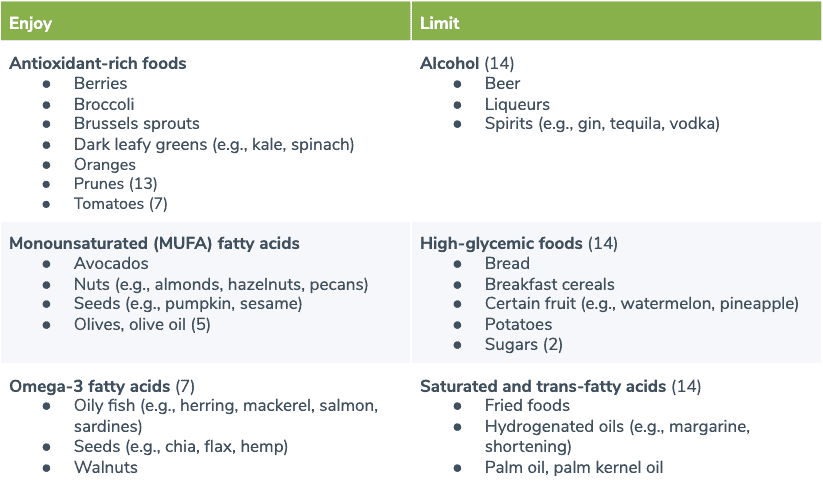
Dietary supplements
Dietary supplements may support mitochondrial function and energy production in various ways. For example, certain supplements may provide nutrients used for oxidative phosphorylation, reduce oxidative stress, encourage the generation of new mitochondria, and repair damaged cell membranes that may be interrupting the transfer of electrons. (8)
Several dietary supplements may be beneficial due to their roles in energy production, including:
- Alpha lipoic acid (ALA), an antioxidant and cofactor in mitochondrial energy production (15)
- B vitamins, required as cofactors for enzymes in cellular function and energy production (8)
- Coenzyme Q10 (CoQ10), an antioxidant and cofactor in the electron transport chain (11)
- L-carnitine, involved in the transport of fatty acids into the mitochondria to be used as energy (8)
- Acetyl-l-carnitine (ALCAR), a form of l-carnitine, which may be better absorbed and cross the blood-brain barrier (8)
- Magnesium, which optimizes mitochondrial function and is used in ATP production (8)
- Vitamin C, an antioxidant, which may preserve mitochondrial membrane potential and may improve mitochondrial function by providing hydrogen and supporting electron transport (8)
- Vitamin E, an antioxidant, which protects cell membranes from lipid oxidation (12)

Regular physical activity
Exercise has been shown to induce mitochondria biogenesis, the process by which new mitochondria are produced. A research panel appointed by the Mitochondrial Medicine Society recommends engaging in endurance exercise, which can increase the activity of mitochondrial enzymes in muscles. (13) Examples of endurance exercises include climbing stairs, biking, dancing, jogging, swimming, and walking.
For individuals with mitochondrial disease, a combination of progressive resistance exercise may be beneficial. (13) Resistance exercises may include weight lifting, resistance band training, and body weight exercises (e.g., pushups, squats).
Energy production is a multi-step process that occurs primarily in the mitochondria found in cells.Minimize exposure to environmental toxins
Many environmental factors have been established as mitochondrial toxins. These toxins induce mitochondrial oxidative stress, which may be associated with an increased risk of neurodegenerative conditions, including Alzheimer’s disease and Parkinson’s disease. Environmental toxins associated with mitochondrial dysfunction include pesticides (e.g., Maneb, Paraquat, Rotenone) and heavy metals (e.g., aluminum, lead, manganese, methylmercury). (19) Minimize your exposure to pesticides by choosing organic foods as much as possible, carefully washing produce, and avoiding the use of pesticides in the garden. Installing a water filter can help remove heavy metals commonly found in tap water. (10) The Natural Resources Defense Council has developed a guide outlining seafood types with low, moderate, and high mercury levels that can be used to minimize consumption of mercury from seafood. (11) Use the Environmental Working Group’s Cosmetics Database (6) and Guide to Healthy Cleaning (5) to identify personal care and household products that are safe for your health and the environment.The bottom line
Energy production is a multi-step process that occurs primarily in the mitochondria found in cells. Improving your energy levels may involve multiple factors, such as dietary modifications, dietary supplements, regular exercise, and reduced exposure to environmental toxins. If you are a patient, speak with your integrative healthcare practitioner about incorporating these changes into your wellness plan.- Alberts, B., Johnson, A., & Lewis, J. (2002). How cells obtain energy from food. In Molecular Biology of the Cell. New York, NY: Garland Science. Retrieved from https://www.ncbi.nlm.nih.gov/books/NBK26882/
- Atkinson, F. S., Foster-Powell, K., & Brand-Miller, J. C. (2008). International tables of glycemic index and glycemic load values: 2008. Diabetes care, 31(12), 2281–2283.
- de Nava, A. S. L., & Raja, A. (2019). Physiology, metabolism. In StatPearls . Treasure Island, FL: StatPearls Publishing. Retrieved from https://www.ncbi.nlm.nih.gov/books/NBK546690/
- Douglas Harper. (n.d.). Energy. Online Etymology Dictionary. Retrieved from https://www.etymonline.com/word/energy
- Environmental Working Group. (n.d.). EWG’s Guide To Healthy Cleaning. Retrieved from https://www.ewg.org/guides/cleaners
- Environmental Working Group. (n.d.). EWG Skin Deep® Cosmetics Database. Retrieved from https://www.ewg.org/skindeep/
- FDA. (n.d.). Monounsaturated and polyunsaturated fat. Retrieved from http://www.fda.gov/nutritioneducation
- Gonzalez, M. J., Seyfried, T., Nicolson, G. L., Barclay, B. J., Matta, J., Vasquez, A., … Cintrón, A. (2018). Mitochondrial correction: A new therapeutic paradigm for cancer and degenerative diseases. Journal of Orthomolecular Medicine, 33(4).
- Haß, U., Herpich, C., & Norman, K. (2019). Anti-inflammatory diets and fatigue. Nutrients, 11(10), 2315.
- Jaishankar, M., Tseten, T., Anbalagan, N., Mathew, B. B., & Beeregowda, K. N. (2014). Toxicity, mechanism and health effects of some heavy metals. Interdisciplinary Toxicology, 7(2), 60–72.
- Natural Resources Defense Council. (2006). Mercury in fish. Retrieved from https://www.nrdc.org/sites/default/files/walletcard.pdf
- NIH. (2018). Dietary supplements for primary mitochondrial disorders. Retrieved from https://ods.od.nih.gov/factsheets/PrimaryMitochondrialDisorders-HealthProfessional/
- Parikh, S., Goldstein, A., Koenig, M. K., Scaglia, F., Enns, G. M., Saneto, R., … DiMauro, S. (2015). Diagnosis and management of mitochondrial disease: A consensus statement from the Mitochondrial Medicine Society. Genetics in Medicine, 17(9), 689–701.
- Rosenthal, T. C., Majeroni, B. A., Pretorius, R, & Malik, Khalid. (2008). Fatigue: An overview. American Family Physician, 78(10), 1173-1179.
- Shay, K. P., Moreau, R. F., Smith, E. J., Smith, A. R., & Hagen, T. M. (2009). Alpha-lipoic acid as a dietary supplement: Molecular mechanisms and therapeutic potential. Biochimica et Biophysica Acta, 1790(10), 1149–1160.
- USDA. (2019). Food and nutrition research briefs. Retrieved from https://www.ars.usda.gov/oc/fnrb/fnrb499/
- Vetrani, C., Costabile, G., Marino, L. D., & Rivellese, A. A. (2012). Nutrition and oxidative stress: A systematic review of human studies. International Journal of Food Sciences and Nutrition, 64(3), 312–326.
- Zick, S. M., Colacino, J., Cornellier, M., Khabir, T., Surnow, K., & Djuric, Z. (2017). Fatigue reduction diet in breast cancer survivors: A pilot randomized clinical trial. Breast Cancer Research and Treatment, 161(2), 299–310.
- Zolkipli-Cunningham, Z., & Falk, M. J. (2017). Clinical effects of chemical exposures on mitochondrial function. Toxicology, 391, 90–99.





