Wellness blog
Featured articles

News
—Let’s Make Healthcare Whole
Learn how Fullscript is making whole person care more attainable, scalable, and impactful.

Blog
—Benefits of Dietary Supplements: 5 Top Advantages of Dietary & Food Supplements Explained
Healthcare practitioners often incorporate dietary supplements to support better patient care and improve overall health.

Integrative Medicine
—Dietary Supplement Quality Assurance at Fullscript: New Supplement Brands & Product Handling
The focus on quality starts from brand selections for our catalog and does not end until the recommended product gets delivered to your patients.

Blog
—3rd Party Supplement Testing: Third-Party Tested Supplements Certification Explained
Learn more about third-party certifications and the most common certifications you may encounter when shopping for third-party certification supplements.
All blog articles

Accessing Creatine Potency
Creatine supports muscle health and performance, but quality varies. Fullscript’s Quality Program tests creatine products to ensure potency, purity, and safety.

Assessing Collagen Potency
Collagen supports joints, skin, and tissues, but quality varies. Fullscript’s Quality Program tests collagen products to ensure purity, potency, and safety.

Vitamin B3 Potency: A Hidden Variable in Supplementation
Vitamin B3 supports energy and DNA health, but dose inconsistencies pose risks. Fullscript’s Quality Program verifies potency and label accuracy for safety.
Providers top picks

Fake Supplements: How to Tell If Supplements Are Fake or Genuine
Understanding how to spot fake supplements can help ensure you’re getting genuine supplements every time. Learn how to spot fake supplements.

Do Supplements Work?: The Scientific Evidence of Dietary Supplements
People often take dietary supplements to address nutrient gaps in their diets, but do supplements actually work? Read on to learn about the current research!
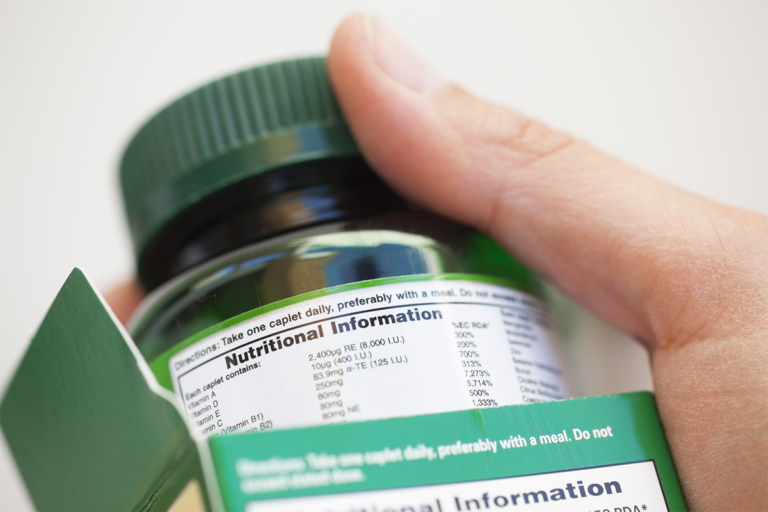
How to Read Supplement Labels: Tips & Guide
Improve your dietary supplement label reading skills. A Guide on how to read supplement labels, facts, and seals.
Research

CCNM’s 6th Annual Research Day (2022)
The Canadian College of Naturopathic Medicine (CCNM) hosted the 6th annual Research Day, bringing together practitioners to discuss and share research findings.

Pediatric Medicine Research Update
This issue includes studies involving infants, children, and adolescents to keep practitioners up to date on impactful research that is relevant to the field of integrative medicine.

Research Update: SCNM’s Fall 2021 Research Night
This article summarizes SCNM Fall 2021 research night featuring presentations on herbal treatment of bovine mastitis and Echinacea and Artemisia in humans.
