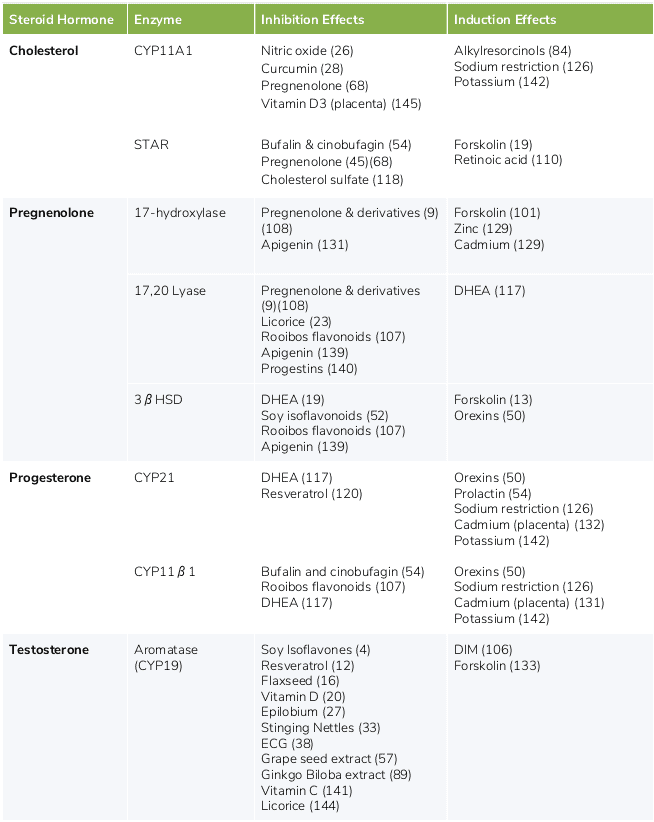
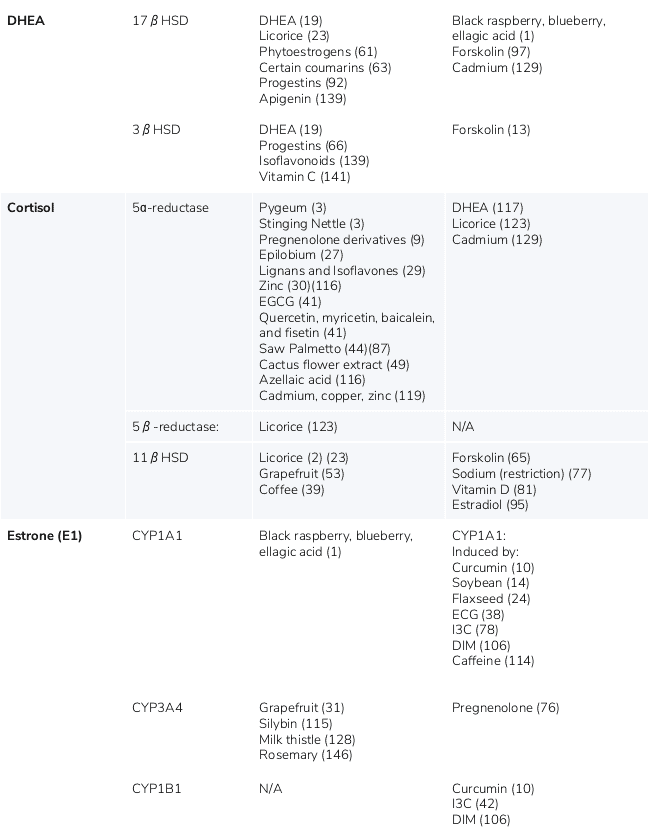
Various foods and nutrients can up- or down-regulate the steroidogenic pathways, resulting in increased or decreased synthesis of steroid hormones. (124) This may occur through a variety of mechanisms, including enzymatic induction or inhibition, and positive or negative feedback mechanisms. (21)(127) There are six major nutrients, botanicals or exogenous hormones that affect steroidogenic hormone levels in humans, including DHEA, pregnenolone, licorice, saw palmetto, Coleus forskohlii, and zinc. Table 1 lists additional ingredients that may affect the steroidogenic pathways.
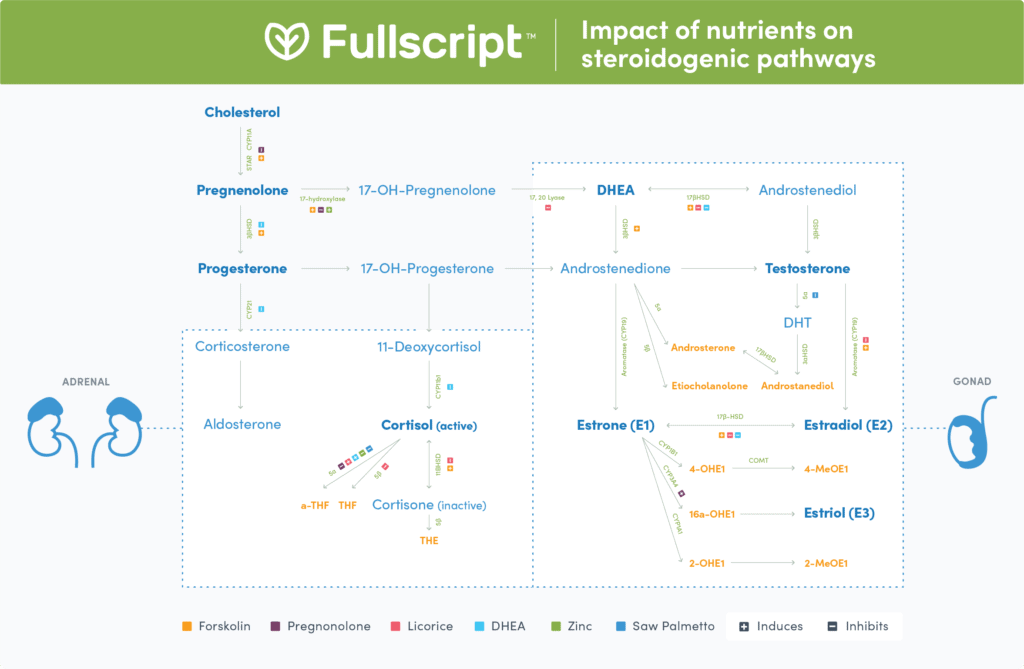
DHEA
Exogenous DHEA has been used to improve symptoms of aging, physical and psychological well-being, strength and body composition, and sexual function. (103) DHEA may induce or inhibit enzymes involved in regulating the steroidogenic pathways. Specifically, DHEA may induce 17,20 lyase and 5ɑ reductase, while it may also inhibit CYP21, CYP11β1, (117) 3βHSD, and 17βHSD. (19) Administration of oral DHEA increases serum DHEA, (79)(143)(22)(51) DHEA-S, (22)(45)(48)(51)(55)(70)(85)(130)(143) testosterone, (22)(45)(55)(70)(85)(143) androstenedione, (18)(22) estradiol, (45)(51)(55)(85) estrone, (45)(55) and insulin-like growth factor-1 (IGF-1). (45) A study with postmenopausal women showed that supplementation over one year with 25 mg of DHEA increases progesterone, DHT, 17-hydroxyprogesterone, allopregnanolone, beta-endorphin, sexual hormone-binding globulin, LH, FSH, growth hormone, and IGF-1 without affecting endometrial thickness. (34) Evidence of the effect of DHEA on cortisol levels is conflicting as cortisol may either increase, (34)(90) or decrease (35)(117) as a result of DHEA intake. Overall, research supports the use of DHEA to improve cognitive and physical function in older women, (55)(143) and in treating hypoadrenalism, (69) infertility, (11)(97)(109)(137) sexual dysfunction, (93) depression, (94) and atherosclerosis. (73)

Pregnenolone
Pregnenolone is a precursor to steroid hormones. (135) Administration of pregnenolone or its derivatives may result in the inhibition of CYP11A1, STAR, (68) 17-hydroxylase, 17,20 lyase, (9)(108) and 5ɑ-reductase. (9) Pregnenolone administration has led to increased levels of pregnenolone, allopregnanolone, pregnenolone sulfate, DHEAS, and progesterone. (74) There is evidence that it may also induce CYP3A4. (76)
Ready to start delivering better patient care?
Pregnenolone is also considered a neurosteroid that increases neuronal activity and may play a major role in neurological conditions. (135) In addition to pregnenolone, other steroids including DHEA, progesterone, and their derivatives can act as neurosteroids and are synthesized by steroidogenic enzymes in the brain. They may regulate gene expression through nuclear receptor binding, modulate neurotransmitter release, and regulate GABA, NMDA, and sigma-1 receptor activities. (138) Pregnenolone, in particular, may improve attention deficits and executive function, (62) as well as working memory (100) and functional capacity (75) in patients with schizophrenia. Additionally, it may be used to treat depressive symptoms in bipolar disorder (17) and improve scores for irritability, lethargy/social withdrawal, and short sensory profile in patients with autism. (32) Evidence exists for its use in treating manic and depressive symptoms in substance-use disorders. (86)
Licorice (Glycyrrhiza glabra)
Licorice has antibacterial, anti-inflammatory, antiviral, antioxidant, and antidiabetic functions. (91) Licorice and its flavonoids mainly act as inhibitors of steroidogenic pathway enzymes, specifically 17,20 lyase, (23) 3βHSD, 11βHSD, (52) 5β, (123) and CYP19. (4) Human trials examining the effects of oral licorice supplementation support its use in alleviating symptoms of menopause including hot flashes, (36)(81) increasing muscle mass, (60) and reducing total fat, visceral fat, and BMI. (6)(71)(125) Licorice administration can decrease testosterone levels (5)(7) and aldosterone, (112) but increase deoxycorticosterone, DHEA, and testosterone. (2) Another study showed that DHEAS decreased in men in response to licorice. (111) Licorice can increase the urinary cortisol/free cortisone quotient. (113) The children of pregnant mothers who had consumed greater than 500 mg per week of glycyrrhizin in licorice during pregnancy also demonstrated higher salivary cortisol concentrations compared with the children of mothers consuming low levels of glycyrrhizin in licorice (less than 250 mg per week). (99) Therefore, high levels of licorice consumption are not recommended during pregnancy due to increased associations with harmful cognitive development in offspring. (98) The consumption of licorice may result in side effects of increased blood pressure, possibly as a consequence of an increased cortisone quotient. (113)
Forskolin (Coleus forskohlii)
Forskolin, a constituent of Coleus forskohlii, may induce many enzymes of the steroidogenic pathways, including STAR, (19) 17-hydroxylase, (101) 3βHSD, (13) 17βHSD, (56) and CYP19. (133) The oral administration of 500 mg (10% extract) per day of forskolin over 12 weeks may weakly increase testosterone levels, but with interindividual variability. (37) Forskolin has also been shown to increase cortisol and DHEA secretion, while increasing 3 beta-HSD and P450c17 expression and activity in human adrenocortical cells. (13) Forskolin can also induce increases in aldosterone. (122) Forskolin may decrease body fat percentage, fat mass and bone mass in conjunction with increased testosterone levels in overweight and obese men. (37) It may also mitigate weight gain in women (40) and treat hypertension. (46)
Saw palmetto (Serenoa repens)
Saw palmetto is well known for its inhibition of 5ɑ-reductase. (87)(136) This may be of particular importance for reducing the conversion of testosterone to DHT, which is linked to benefits seen in treating benign prostatic hyperplasia (BPH) and lower urinary tract symptoms in a similar manner to the pharmaceutical, finasteride. (87) Treatment with saw palmetto has been shown to decrease DHT and epidermal growth factor, and increase testosterone in BPH tissues, suggesting inhibition of 5ɑ-reductase. (25) In vitro studies suggest that saw palmetto’s treatment of BPH may be linked to its inhibition of 5ɑ-reductase. (88)(134) It has been shown to reduce prostate size and improve urinary flow, (104) as well as chronic prostatic inflammation. (64) Saw palmetto extract can also increase average and terminal hair count and thus may have potential in treating male pattern baldness with topical (136) or oral administration. (102) Evidence also suggests that saw palmetto extract can be used in the treatment of prostatitis, (80) sexual dysfunction, (121) and polycystic ovarian syndrome. (15) Doses of up to 960 mg over 18 months have been shown as safe and non-toxic. (8)
Zinc
Zinc has been shown to inhibit 5ɑ-reductase (30)(116)(119) and induce 17-hydroxylase (129) in vitro. In humans, treatment with 10 mg of zinc sulfate (43) or 50 mg of elemental zinc acetate (72) can lead to increased testosterone, DHT levels, and sperm count. (83) Similarly, daily supplementation with 3 mg/kg of body weight of zinc sulfate can prevent decreased testosterone and thyroid hormones associated with exercise in sedentary (58) and athletic men. (59) In male patients with end-stage renal disease on hemodialysis, 250mg per day of supplemental zinc sulfate led to an increase in testosterone and luteinizing hormone. (47) Higher doses of 50 mg three times per day may lead to nausea, vomiting, gastrointestinal cramping, loss of appetite, diarrhea, headaches, lethargy, anemia, and dizziness, (96)(105) while chronic ingestion of greater than 100 mg per day over ten years has been associated with a higher incidence of prostatic cancer. (67)
Additional ingredients that may affect the steroidogenic pathways
The nutrients presented in this table may up-regulate (induce) or down-regulate (inhibit) steroidogenic pathway enzymes, resulting in increased or decreased levels of specific steroid hormones.

The bottom line
Certain nutrients, including DHEA, pregnenolone, licorice, forskolin, saw palmetto, and zinc, have been shown to impact the steroidogenic pathways. These nutrients, which can be found in food and supplements, may induce or inhibit steroidogenic pathway enzymes.
Ready to start delivering better patient care?

- Aiyer, H. S., & Gupta, R. C. (2010). Berries and ellagic acid prevent estrogen-induced mammary tumorigenesis by modulating enzymes of estrogen metabolism. Cancer Prevention Research,3(6), 727-737.
- Al-Dujailia, E.A.S., Kenyon, C.J., Nicol, M.R., & Mason, J.I. (2011). Liquorice and glycyrrhetinic acid increase DHEA and deoxycorticosterone levels in vivo and in vitro by inhibiting adrenal SULT2A1 activity. Molecular and Cellular Endocrinology, 336(1–2), 102-109
- Allkanjari, O., & Vitalone, A. (2015). What do we know about phytotherapy of benign prostatic hyperplasia? Life Sciences,126, 42-56.
- Amaral, C., Toloi, M.R.T., Vasconcelos, L.D., Fonseca, M.J.V., Correia-da-Silva, G., & Teixeira, N. (2017). The role of soybean extracts and isoflavones in hormone-dependent breast cancer: aromatase activity and biological effects. Food & Function, 8(9), 3064-3074.
- Armanini, D., Bonanni, G., Mattarello, M.J., Fiore, C., Sartorato, P., & Palermo, M. (2003). Licorice consumption and serum testosterone in healthy man. Experimental and Clinical Endocrinology & Diabetes, 111(6), 341-3.
- Armanini, D., De Palo, C.B., Mattarello, M.J., Spinella, P., Zaccaria, M., Ermolao, A., … Karbowiak, I. (2003). Effect of licorice on the reduction of body fat mass in healthy subjects. Journal of Endocrinological Investigation, 26(7), 646-50.
- Armanini, D., Mattarello, M.J., Fiore, C., Bonanni, G., Scaroni, C., Sartorato, P., & Palermo, M. (2004). Licorice reduces serum testosterone in healthy women. Steroids, 69(11-12), 763-6.
- Avins, A.L., Lee, J.Y., Meyers, C.M., & Barry, M.J. (2013). Safety and toxicity of saw palmetto in the Complementary and Alternative Medicine for Urological Symptoms (CAMUS) Trial. The Journal of Urology, 189(4), 1415–1420.
- Banday, A.H., Shameem, S.A., Banday, J.A., & Ganaie, B.A. (2018). Synthesis, 17α-hydroxylase-C17,20-lyase inhibitory and 5AR reductase activity of novel pregnenolone derivatives. Anti-Cancer Agents in Medicinal Chemistry, 18(13), 1919-1926.
- Bansal, S. S., Kausar, H., Vadhanam, M. V., Ravoori, S., Pan, J., Rai, S. N., & Gupta, R. C. (2014). Curcumin implants, not curcumin diet, inhibit estrogen-induced mammary carcinogenesis in ACI rats. Cancer Prevention Research,7(4), 456-465.
- Barad, D., & Gleicher, N. (2006). Effect of dehydroepiandrosterone on oocyte and embryo yields, embryo grade and cell number in IVF. Human Reproduction, 21(11), 2845-9.
- Baravalle, R., Ciaramella, A., Baj, F., Nardo, G. D., & Gilardi, G. (2018). Identification of endocrine disrupting chemicals acting on human aromatase. Biochimica Et Biophysica Acta (BBA) – Proteins and Proteomics,1866(1), 88-96.
- Bird, I.M., Imaishi, K., Pasquarette, M.M., Rainey, W.E., & Mason, J.I. (1996). Regulation of 3 beta-hydroxysteroid dehydrogenase expression in human adrenocortical H295R cells. Journal of Endocrinology, 50 Suppl, S165-73.
- Bogacz, A., Bartkowiak-Wieczrek, J., Mikołajczak, P., Rakowska-Mrozikiewicz, B., Grześkowiak, E., Wolski, H., . . . Mrozikiewicz, P. (2014). The influence of soybean extract on the expression level of selected drug transporters, transcription factors and cytochrome P450 genes encoding phase I drug-metabolizing enzymes. Polish Gynaecology,85(5).
- Bongaard, B.S. (2015). Polycystic ovary syndrome. In V. Maizes, & T. Low Dog (Eds,). Integrative women’s health (289-302). Retrieved from https://books.google.ca/books?hl=en&lr=&id=uveJCgAAQBAJ&oi=fnd&pg=PA289&dq=saw+palmetto+polycystic+ovarian+syndrome&ots=V1VCXdvEiE&sig=S7hDKlx8MXhAvMrHj85d4Bt6whE#v=onepage&q=saw%20palmetto%20polycystic%20ovarian%20syndrome&f=false
- Brooks, J. D., & Thompson, L. U. (2005). Mammalian lignans and genistein decrease the activities of aromatase and 17β-hydroxysteroid dehydrogenase in MCF-7 cells. The Journal of Steroid Biochemistry and Molecular Biology,94(5), 461-467.
- Brown, E.S., Park, J., Marx, C.E., Hynan, L.S., Gardner, C., Davila, D., … Holmes, T. (2014). A randomized, double-blind, placebo-controlled trial of pregnenolone for bipolar depression. Neuropsychopharmacology, 39(12), 2867-73.
- Brown, G.A., Vukovich, M.D., Sharp, R.L., Reifenrath, T.A., Parsons, K.A., & King, D.S. (1985). Effect of oral DHEA on serum testosterone and adaptations to resistance training in young men. The Journal of Applied Physiology, 87(6), 2274-83.
- Chen, W., Tsai, S.J., Sheu, H.M., Tsai, J.C., & Zouboulis, C.C. (2010). Testosterone synthesized in cultured human SZ95 sebocytes derives mainly from dehydroepiandrosterone. Experimental Dermatology, 19(5), 470-2.
- Cutolo, M., Paolino, S., Sulli, A., Smith, V., Pizzorni, C., & Seriolo, B. (2014). Vitamin D, steroid hormones, and autoimmunity. Annals of the New York Academy of Sciences,1317(1), 39-46.
- Darney, K.J.Jr., Zirkin, B.R., & Ewing, L.L. (1996). Testosterone autoregulation of its biosynthesis in the rat testis: inhibition of 17 alpha-hydroxylase activity. Journal of Andrology, 17(2), 137-42.
- Dayal, M., Sammel, M.D., Zhao, J., Hummel, A.C., Vandenbourne, K., & Barnhart, K.T. (2005). Supplementation with DHEA: effect on muscle size, strength, quality of life, and lipids. Journal of Women’s Health, 14(5), 391-400.
- Deluca, D., Krazeisen, A., Breitling, R., Prehn, C., Moller, G., & Adamski, J. (2005). Inhibition of 17beta-hydroxysteroid dehydrogenases by phytoestrogens: Comparison with other steroid metabolizing enzymes. Journal of Steroid Biochemistry & Molecular Biology, 93(2005), 285–292.
- Dikshit, A., Hales, K., & Hales, D. B. (2017). Whole flaxseed diet alters estrogen metabolism to promote 2-methoxtestradiol-induced apoptosis in hen ovarian cancer. The Journal of Nutritional Biochemistry,42, 117-125.
- Di Silverio, F., Monti, S., Sciarra, A., Varasano, P.A., Martini, C., Lanzara, S., & … Toscano, V. (1998). Effects of long-term treatment with Serenoa repens (Permixon) on the concentrations and regional distribution of androgens and epidermal growth factor in benign prostatic hyperplasia. Prostate, 37(2), 77-83.
- Drewett, J. G., Adams-Hays, R. L., Ho, B. Y., & Hegge, D. J. (2002). Nitric oxide potently inhibits the rate-limiting enzymatic step in steroidogenesis. Molecular and Cellular Endocrinology,194(1-2), 39-50.
- Ducrey, B., Marston, A., Göhring, S., Hartmann, R., & Hostettmann, K. (1997). Inhibition of 5α-reductase and aromatase by the ellagitannins oenothein A and oenothein B from Epilobium species. Planta Medica,63(2), 111-114.
- Enyeart, J. A., Liu, H., & Enyeart, J. J. (2009). Curcumin inhibits ACTH- and angiotensin II-stimulated cortisol secretion and Cav3.2 current. Journal of Natural Products,72(8), 1533-1537.
- Evans, B. A., Griffiths, K., & Morton, M. S. (1995). Inhibition of 5α-reductase in genital skin fibroblasts and prostate tissue by dietary lignans and isoflavonoids. Journal of Endocrinology,147(2), 295-302.
- Fahim, M.S., Wang, M., Sutcu, M.F., & Fahim, Z. (1993). Zinc arginine, a 5 alpha-reductase inhibitor, reduces rat ventral prostate weight and DNA without affecting testicular function. Andrologia, 25(6), 369-75.
- Fukuda, K., Ohta, T., Oshima, Y., Ohashi, N., Yoshikawa, M., & Yamazoe, Y. (1997). Specific CYP3 A4 inhibitors in grapefruit juice: Furocoumarin dimers as components of drug interaction. Pharmacogenetics,7(5), 391-396.
- Fung, L.K., Libove, R.A., Phillips, J., Haddad, F., & Hardan, A.Y. (2014). Brief report: An open-label study of the neurosteroid pregnenolone in adults with autism spectrum disorder. Journal of Autism and Developmental Disorders, 44(11), 2971–2977.
- Gansser, D., & Spiteller, G. (1995). Aromatase inhibitors from Urtica dioica roots. Planta Medica,61(2), 138-140.
- Genazzani, A.D., Stomati, M., Bernardi, F., Pieri, M., Rovati, L., & Genazzani, A.R. (2003). Long-term low-dose dehydroepiandrosterone oral supplementation in early and late postmenopausal women modulates endocrine parameters and synthesis of neuroactive steroids. Fertility and Sterility, 80(6), 1495-501.
- Genazzani, A.R., Pluchino, N., Begliuomini, S., Stomati, M., Bernardi, F., Pieri, M., … Luisi, M. (2006). Long-term low-dose oral administration of dehydroepiandrosterone modulates adrenal response to adrenocorticotropic hormone in early and late postmenopausal women. Gynecological Endocrinology, 22(11), 627-35.
- Ghazanfarpour, M., Sadeghi, R., Abdolahian, S., & Latifnejad Roudsari, R. (2016). The efficacy of Iranian herbal medicines in alleviating hot flashes: A systematic review. International Journal of Reproductive BioMedicine, 14(3), 155–166.
- Godard, M.P., Johnson, B.A., & Richmond, S.R. (2005). Body composition and hormonal adaptations associated with forskolin consumption in overweight and obese men. Obesity Research, 13(8), 1335-43.
- Goodin, M., Bray, B., & Rosengren, R. (2006). Sex- and strain-dependent effects of epigallocatechin gallate (EGCG) and epicatechin gallate (ECG) in the mouse. Food and Chemical Toxicology,44(9), 1496-1504.
- Gumy, C., Thurnbichler, C., Aubry, E. M., Balazs, Z., Pfisterer, P., Baumgartner, L., . . . Rollinger, J. M. (2009). Inhibition of 11β-hydroxysteroid dehydrogenase type 1 by plant extracts used as traditional antidiabetic medicines. Fitoterapia,80(3), 200-205.
- Henderson, S., Magu, B., Rasmussen, C., Lancaster, S., Kerksick, C., Smith, P., … Kreider, R.B. (2005). Effects of coleus forskohlii supplementation on body composition and hematological profiles in mildly overweight women. Journal of the International Society of Sports Nutrition, 2, 54-62.
- Hiipakka, R. A., Zhang, H., Dai, W., Dai, Q., & Liao, S. (2002). Structure–activity relationships for inhibition of human 5α-reductases by polyphenols. Biochemical Pharmacology, 63(6), 1165-1176.
- Horn, T. L., Reichert, M. A., Bliss, R. L., & Malejka-Giganti, D. (2002). Modulations of P450 mRNA in liver and mammary gland and P450 activities and metabolism of estrogen in liver by treatment of rats with indole-3-carbinol. Biochemical Pharmacology,64(3), 393-404.
- Hunt, C.D., Johnson, P.E., Herbel, J., & Mullen, L.K. (1992). Effects of dietary zinc depletion on seminal volume and zinc loss, serum testosterone concentrations, and sperm morphology in young men. American Journal of Clinical Nutrition, 56(1), 148-57.
- Iehlé, C., Délos, S., Guirou, O., Tate, R., Raynaud, J., & Martin, P. (1995). Human prostatic steroid 5α-reductase isoforms—A comparative study of selective inhibitors. The Journal of Steroid Biochemistry and Molecular Biology,54(5-6), 273-279.
- Igwebuike, A., Irving, B.A., Bigelow, M.L., Short, K.R., McConnell, J.P., & Nair, K.S. (2008). Lack of dehydroepiandrosterone effect on a combined endurance and resistance exercise program in postmenopausal women. The Journal of Clinical Endocrinology and Metabolism, 93(2), 534-8.
- Jagtap, M., Chandola, H.M., & Ravishankar, B. (2011). Clinical efficacy of Coleus forskohlii (Willd.) Briq. (Makandi) in hypertension of geriatric population. Ayu, 32(1), 59-65.
- Jalali, G.R., Roozbeh, J., Mohammadzadeh, A., Sharifian, M., Sagheb, M.M., Hamidian Jahromi, A., … Afshariani, R. (2010). Impact of oral zinc therapy on the level of sex hormones in male patients on hemodialysis. Renal Failure, 32(4), 417-9.
- Jedrzejuk, D., Medras, M., Milewicz, A., & Demissie, M. (2003). Dehydroepiandrosterone replacement in healthy men with age-related decline of DHEA-S: effects on fat distribution, insulin sensitivity and lipid metabolism. Aging Male, 6(3), 151-6.
- Jonas, A., Rosenblat, G., Krapf, D., Bitterman, W., & Neeman, I. (1998). Cactus flower extracts may prove beneficial in benign prostatic hyperplasia due to inhibition of 5α reductase activity, aromatase activity and lipid peroxidation. Urological Research,26(4), 265-270.
- Kagerer, S. M., Eichholz, C., & Jöhren, O. (2011). Orexins/hypocretins increase the promoter activity of selective steroidogenic enzymes. Peptides,32(4), 839-843.
- Kahn, A.J., Halloran, B., Wolkowitz, O., & Brizendine, L. (2002). Dehydroepiandrosterone supplementation and bone turnover in middle-aged to elderly men. Journal of Clinical Endocrinology and Metabolism, 87(4), 1544-9.
- Kaminska, B., Ciereszko, R., Kiezun, M., & Dusza, L. (2013). In vitro effects of genistein and daidzein on the activity of adrenocortical steroidogenic enzymes in mature female pigs. Journal of Physiology and Pharmacology, 64(1), 103-8.
- Kargl, C., Arshad, M., Salman, F., Schurman, R. C., & Corral, P. D. (2017). 11β-hydroxysteroid dehydrogenase type-II activity is affected by grapefruit juice and intense muscular work. Archives of Endocrinology and Metabolism,61(6), 556-561.
- Kau, M., Wang, J., Tsai, S., Yu, C., & Wang, P. S. (2012). Inhibitory effect of bufalin and cinobufagin on steroidogenesis via the activation of ERK in human adrenocortical cells. British Journal of Pharmacology,165(6), 1868-1876.
- Kenny, A.M., Boxer, R.S., Kleppinger, A., Brindisi, J., Feinn, R., & Burleson, J.A. (2010). Dehydroepiandrosterone combined with exercise improves muscle strength and physical function in frail older women. Journal of the American Geriatrics Society, 58(9), 1707-14.
- Khanum, A., Buczko, E., & Dufau, M.L. (1997). Essential role of adenosine triphosphate in activation of 17beta-hydroxysteroid dehydrogenase in the rat Leydig cell. Endocrinology, 138(4), 1612-20.
- Kijima, I., Phung, S., Hur, G., Kwok, S., & Chen, S. (2006). Grape seed extract is an aromatase inhibitor and a suppressor of aromatase expression. Cancer Research,66(11), 5960-5967.
- Kilic, M. (2007). Effect of fatiguing bicycle exercise on thyroid hormone and testosterone levels in sedentary males supplemented with oral zinc. Neuroendocrinology Letters, 28(5), 681-5.
- Kilic, M., Baltaci, A.K., Gunay, M., Gökbel, H., Okudan, N., & Cicioglu, I. (2006). The effect of exhaustion exercise on thyroid hormones and testosterone levels of elite athletes receiving oral zinc. Neuroendocrinology Letters, 27(1-2), 247-52.
- Kinoshita, T., Matsumoto, A., Yoshino, K., & Furukawa, S. (2017). The effects of licorice flavonoid oil with respect to increasing muscle mass: a randomized, double-blind, placebo-controlled trial. Journal of the Science of Food and Agriculture, 97(8), 2339-2345.
- Krazeisen, A., Breitling, R., Möller, G., & Adamski, J. (2002). Human 17β-hydroxysteroid dehydrogenase type 5 is inhibited by dietary flavonoids. Flavonoids in Cell Function Advances in Experimental Medicine and Biology,505, 151-161.
- Kreinin, A., Bawakny, N., & Ritsner, M.S. (2017). Adjunctive pregnenolone ameliorates the cognitive deficits in recent-onset schizophrenia: An 8-week, randomized, double-blind, placebo-controlled trial. Clinical Schizophrenia & Related Psychoses, 10(4), 201-210.
- Lain, R. L., Barrell, K., Saeed, G., Nicholls, P., Simons, C., Kirby, A., & Smith, H. (2002). Some coumarins and triphenylethene derivatives as inhibitors of human testes microsomal 17β-hydroxysteroid dehydrogenase (17β-HSD type 3): Further studies with tamoxifen on the rat testes microsomal enzyme. Journal of Enzyme Inhibition and Medicinal Chemistry,17(2), 93-100.
- Latil, A., Pétrissans, M., Rouquet, J., Robert, G., & de la Taille, A. (2015). Effects of hexanic extract of serenoa repens (permixon® 160 mg) on inflammation biomarkers in the treatment of lower urinary tract symptoms related to benign prostatic hyperplasia. Prostate, 75(16), 1857–1867.
- Lecybyl, R., Rubis, B., Krozowski, Z., & Trzeciak, W. H. (2003). Regulation of 11β‐hydroxysteroid dehydrogenase type II expression in the renal epithelial cells. Endocrine Research,29(2), 211-216.
- Lee, T. C., Miller, W. L., & Auchus, R. J. (1999). Medroxyprogesterone acetate and dexamethasone are competitive inhibitors of different human steroidogenic enzymes. The Journal of Clinical Endocrinology & Metabolism, 84(6), 2104-2110.
- Leitzmann, M.F., Stampfer, M.J., Wu, K., Colditz, G.A., Willett, W.C., & Giovannucci, E.L. (2003). Zinc supplement use and risk of prostate cancer. Journal of the National Cancer Institute, 95(13), 1004-7.
- Li, J., Zhou, Q., Ma, Z., Wang, M., Shen, W.J., Azhar, S., … Hu, Z. (2017). Feedback inhibition of CREB signaling by p38 MAPK contributes to the negative regulation of steroidogenesis. Reproductive Biology and Endocrinology, 15(1), 19.
- Libè, R., Barbetta, L., Dall’Asta, C., Salvaggio, F., Gala, C., Beck-Peccoz, P., & Ambrosi, B. (2004). Effects of dehydroepiandrosterone (DHEA) supplementation on hormonal, metabolic and behavioral status in patients with hypoadrenalism. Journal of Endocrinological Investigation, 27(8), 736-41.
- Liu, T.C., Lin, C.H., Huang, C.Y., Ivy, J.L., & Kuo, C.H. (2013). Effect of acute DHEA administration on free testosterone in middle-aged and young men following high-intensity interval training. European Journal of Applied Physiology, 113(7), 1783-92.
- Luís, ., Domingues, F., & Pereira, L. (2018). Metabolic changes after licorice consumption: A systematic review with meta-analysis and trial sequential analysis of clinical trials. Phytomedicine, 39, 17-24.
- Mahajan, S.K., Abbasi, A.A., Prasad, A.S., Rabbani, P., Briggs, W.A., & McDonald, F.D. (1982). Effect of oral zinc therapy on gonadal function in hemodialysis patients. A double-blind study. Annals of Internal Medicine, 97(3), 357-61.
- Martina, V., Benso, A., Gigliardi, V.R., Masha, A., Origlia, C., Granata, R., & Ghigo, E. (2006). Short-term dehydroepiandrosterone treatment increases platelet cGMP production in elderly male subjects. Clinical Endocrinology, 64(3), 260-4.
- Marx, C.E., Keefe, R.S.E., Buchanan, R.W., Hamer, R.M., Kilts, J.D., Bradford, D.W., … Shampine, L.J. (2009). Proof-of-concept trial with the neurosteroid pregnenolone targeting cognitive and negative symptoms in schizophrenia. Neuropsychopharmacology, 34(8), 1885–1903.
- Marx, C.E., Lee, J., Subramaniam, M., Rapisarda, A., Bautista, D.C., Chan, E., … Chong, S.A. (2014). Proof-of-concept randomized controlled trial of pregnenolone in schizophrenia. Psychopharmacology (Berl), 231(17), 3647-62.
- Masuyama, H., Nakamura, K., Nobumoto, E., & Hiramatsu, Y. (2016). Inhibition of pregnane X receptor pathway contributes to the cell growth inhibition and apoptosis of anticancer agents in ovarian cancer cells. International Journal of Oncology, 49(3), 1211-20.
- Mckinnell, J., Roscoe, D., Holmes, M. C., Lloyd-Macgilp, S. A., & Kenyon, C. J. (2000). Regulation of 11β-hydroxysteroid dehydrogenase enzymes by dietary sodium in the rat. Endocrine Research,26(1), 81-95.
- Michnovicz, J. J., & Bradlow, H. L. (1990). Induction of estradiol metabolism by dietary indole-3-carbinol in humans. JNCI Journal of the National Cancer Institute,82(11), 947-949.
- Morales, A., Black, A., Emerson, L., Barkin, J., Kuzmarov, I., & Day, A. (2009). Androgens and sexual function: a placebo-controlled, randomized, double-blind study of testosterone vs. dehydroepiandrosterone in men with sexual dysfunction and androgen deficiency. Aging Male, 12(4), 104-12.
- Morgia, G., Mucciardi, G., Galì, A., Madonia, M., Marchese, F., Di Benedetto, A., …Magno, C. (2010). Treatment of chronic prostatitis/chronic pelvic pain syndrome category IIIA with Serenoa repens plus selenium and lycopene (Profluss) versus S. repens alone: an Italian randomized multicenter-controlled study. Urologia Internationalis, 84(4), 400-6.
- Morris, K. L., & Zemel, M. B. (2005). 1, 25-dihydroxyvitamin D3 modulation of adipocyte glucocorticoid function. Obesity Research,13(4), 670-677.
- Nahidi, F., Zare, E., Mojab, F., & Alavi-majd, H. Effects of licorice on relief and recurrence of menopausal hot flashes. Iranian Journal of Pharmaceutical Research, 11(2), 541–548.
- Netter, A., Hartoma, R., & Nahoul, K. (1981). Effect of zinc administration on plasma testosterone, dihydrotestosterone, and sperm count. Archives of Andrology, 7(1), 69-73.
- Oskarsson, A., & Andersson, Å O. (2016). Suppressed sex hormone biosynthesis by alkylresorcinols: A possible link to chemoprevention. Nutrition and Cancer,68(6), 978-987.
- Ostojic, S.M., Calleja, J., & Jourkesh, M. (2010). Effects of short-term dehydroepiandrosterone supplementation on body composition in young athletes. Chinese Journal of Physiology, 53(1), 19-25.
- Osuji, I.J., Vera-Bolaños, E., Carmody, T.J., & Brown, E.S. (2010). Pregnenolone for cognition and mood in dual diagnosis patients. Psychiatry Research, 178(2), 309-12.
- Pais, P. (2010). Potency of a novel saw palmetto ethanol extract, SPET-085, for inhibition of 5alpha-reductase II. Advances in Therapy, 27(8), 555-63.
- Palin, M.F., Faguy, M., LeHoux, J.G., & Pelletier, G. (1998). Inhibitory effects of Serenoa repens on the kinetic of pig prostatic microsomal 5alpha-reductase activity. Endocrine, 9(1), 65-9.
- Park, Y. J., Choo, W. H., Kim, H. R., Chung, K. H., & Oh, S. M. (2015). Inhibitory aromatase effects of flavonoids from ginkgo biloba extracts on estrogen biosynthesis. Asian Pacific Journal of Cancer Prevention,16(15), 6317-6325.
- Parsons, T.D., Kratz, K.M., Thompson, E., Stanczyk, F.Z., Buckwalter, J.G. (2006). Dhea supplementation and cognition in postmenopausal women. International Journal of Neuroscience, 116(2), 141-55.
- Pastorino, G., Cornara, L., Soares, S., Rodrigues, F., & Oliveira, M.B.P.P. (2018). Liquorice (Glycyrrhiza glabra): A phytochemical and pharmacological review. Phytotherapy Research, 32(12), 2323-2339.
- Pasqualini, J. R., Ebert, C., & Chetrite, G. S. (2001). Biological effects of progestins in breast cancer. Gynecological Endocrinology,15(6), 44-52.
- Peixoto, C., Carrilho, C.G., Barros, J.A., Ribeiro, T.T., Silva, L.M., Nardi, A.E., … Veras, A.B. (2017). The effects of dehydroepiandrosterone on sexual function: a systematic review. Climacteric, 20(2), 129-137.
- Peixoto, C., Grande, A.J., Mallmann, M.B., Nardi, A.E., Cardoso, A., & Veras, A.B. (2018). Dehydroepiandrosterone (DHEA) for depression: A systematic review and meta-analysis. CNS & Neurological Disorders – Drug Targets, 17(9), 706-711.
- Pepe, G. J., Davies, W. A., Dong, K., Luo, H., & Albrecht, E. D. (1999). Cloning of the 11β-hydroxysteroid dehydrogenase (11β-HSD)-2 gene in the baboon: Effects of estradiol on promoter activity of 11β-HSD-1 and -2 in placental JEG-3 cells. Biochimica Et Biophysica Acta (BBA) – Gene Structure and Expression,1444(1), 101-110.
- Plum, L.M., Rink, L., & Haase, H. (2010). The essential toxin: Impact of zinc on human health. International Journal of Environmental Research and Public Health, 7(4), 1342–1365.
- Qin, J.C., Fan, L., Qin, A.P. (2017). The effect of dehydroepiandrosterone (DHEA) supplementation on women with diminished ovarian reserve (DOR) in IVF cycle: Evidence from a meta-analysis. Journal of Gynecology Obstetrics and Human Reproduction, 46(1), 1-7.
- Räikkönen, K., Martikainen, S., Pesonen, A.K., Lahti, J., Heinonen, K., Pyhälä, R., … Kajantie, E. (2017). Maternal licorice consumption during pregnancy and pubertal, cognitive, and psychiatric outcomes in children. American Journal of Epidemiology, 185(5), 317-328.
- Räikkönen, K., Seckl, J.R., Heinonen, K., Pyhälä, R., Feldt, K., Jones, A., … Kajantie, E. (2010). Maternal prenatal licorice consumption alters hypothalamic-pituitary-adrenocortical axis function in children. Psychoneuroendocrinology, 35(10),1587-93.
- Ritsner, M.S., Gibel, A., Shleifer, T., Boguslavsky, I., Zayed, A., Maayan, R., … Lerner, V. (2010). Pregnenolone and dehydroepiandrosterone as an adjunctive treatment in schizophrenia and schizoaffective disorder: an 8-week, double-blind, randomized, controlled, 2-center, parallel-group trial. The Journal of Clinical Psychiatry, 71(10), 1351-62.
- Roelofs, M.J., Piersma, A.H., van den Berg, M., & van Duursen, M.B. (2013). The relevance of chemical interactions with CYP17 enzyme activity: assessment using a novel in vitro assay. Toxicology and Applied Pharmacology, 268(3), 309-17.
- Rossi, A., Mari, E., Scarno, M., Garelli, V., Maxia, C., Scali, E., … Carlesimo, M. (2012). Comparitive effectiveness of finasteride vs Serenoa repens in male androgenetic alopecia: a two-year study. International Journal of Immunopathology and Pharmacology, 25(4), 1167-73.
- Rutkowski, K., Sowa, P., Rutkowska-Talipska, J., Kuryliszyn-Moskal, A., & Rutkowski, R. (2014). Dehydroepiandrosterone (DHEA): hypes and hopes. Drugs, 74(11), 1195-207.
- Saidi, S., Stavridis, S., Stankov, O., Dohcev, S., & Panov, S. (2017). Effects of Serenoa repens alcohol extract on benign prostate hyperplasia. Pril (Makedon Akad Nauk Umet Odd Med Nauki), 38(2), 123-129.
- Samman, S., & Roberts, D.C. (1987). The effect of zinc supplements on plasma zinc and copper levels and the reported symptoms in healthy volunteers. The Medical Journal of Australia, 146(5), 246-9.
- Sanderson, J. T., Slobbe, L., Lansbergen, G. W., Safe, S., & Van den Berg, M. (2001). 2,3,7,8-Tetrachlorodibenzo-p-dioxin and diindolylmethanes differentially induce cytochrome P450 1A1, 1B1, and 19 in H295R human adrenocortical carcinoma cells. Toxicological Sciences,61(1), 40-48.
- Schloms, L., & Swart, A. (2014). Rooibos flavonoids inhibit the activity of key adrenal steroidogenic enzymes, modulating steroid hormone levels in H295R cells. Molecules,19(3), 3681-3695.
- Schoonen, W.G., Lambert, J.G., Resink, J.W., Viveen, W.J., & Van Oordt, P.G. (1987). A quantitative study of steroid bioconversions in the testis of the African catfish, Clarias gariepinus (Burchell), under natural spawning and natural and cultivated non-spawning conditions. Journal of Endocrinology, 112(2), 323-32.
- Schwarze, J.E., Canales, J., Crosby, J., Ortega-Hrepich, C., Villa, S., & Pommer, R. (2018). DHEA use to improve likelihood of IVF/ICSI success in patients with diminished ovarian reserve: A systematic review and meta-analysis. JBRA Assisted Reproduction, 22(4), 369-374.
- Sesta, A., Cassarino, M. F., Tapella, L., Castelli, L., Cavagnini, F., & Giraldi, F. P. (2016). Effect of retinoic acid on human adrenal corticosteroid synthesis. Life Sciences,151, 277-280.
- Sigurjonsdottir, H.A., Axelson, M., Johannsson, G., Manhem, K., Nystrom, E., & Wallerstedt, S. (2006). Liquorice in moderate doses does not affect sex steroid hormones of biological importance although the effect differs between the genders. Hormone Research in Paediatrics, 65(2), 106-10.
- Sigurjonsdottir, H.A., Axelson, M., Johannsson, G., Manhem, K., Nyström, E., & Wallerstedt, S. (2006). The liquorice effect on the RAAS differs between the genders. Blood Pressure, 15(3), 169-72.
- Sigurjonsdottir, H.A., Manhem, K., Axelson, M., & Wallerstedt, S. (2003). Subjects with essential hypertension are more sensitive to the inhibition of 11 beta-HSD by liquorice. Journal of Human Hypertension, 17(2), 125-31.
- Sowers, M. R., Crawford, S., Mcconnell, D. S., Randolph, J. F., Gold, E. B., Wilkin, M. K., & Lasley, B. (2006). Selected diet and lifestyle factors are associated with estrogen metabolites in a multiracial/ethnic population of women. The Journal of Nutrition,136(6), 1588-1595.
- Sridar, C., Goosen, T. C., Kent, U. M., Williams, J. A., & Hollenberg, P. F. (2004). Silybin inactivates cytochromes P450 3A4 and 2C9 and inhibits major hepatic glucuronosyltransferases. Drug Metabolism and Disposition,32(6), 587-594.
- Stamatiadis, D., Bulteau-Portois, M.C., & Mowszowicz, I. (1988). Inhibition of 5 alpha-reductase activity in human skin by zinc and azelaic acid. British Journal of Dermatology, 119(5), 627-32.
- Stomati, M., Monteleone, P., Casarosa, E., Quirici, B., Puccetti, S., Bernardi, F., … Genazzani, A.R. (2000). Six-month oral dehydroepiandrosterone supplementation in early and late postmenopause. Gynecological Endocrinology, 14(5), 342-63.
- Sugawara, T., Nomura, E., & Hoshi, N. (2007). Cholesterol sulphate affects production of steroid hormones by reducing steroidogenic acute regulatory protein level in adrenocortical cells. Journal of Endocrinology,195(3), 451-458.
- Sugimoto, Y., López-Solache, I., Labrie, F., & Luu-The, V. (1995). Cations inhibit specifically type I 5 alpha-reductase found in human skin. Journal of Investigative Dermatology, 104(5), 775-8.
- Supornsilchai, V., Svechnikov, K., Seidlova-Wuttke, D., Wuttke, W., & Söder, O. (2005). Phytoestrogen resveratrol suppresses steroidogenesis by rat adrenocortical cells by inhibiting cytochrome P450 c21-hydroxylase. Hormone Research in Paediatrics,64(6), 280-286.
- Suter, A., Saller, R., Riedi, E., & Heinrich M. (2013). Improving BPH symptoms and sexual dysfunctions with a saw palmetto preparation? Results from a pilot trial. Phytotherapy Research, 27(2), 218-26.
- Swart, A.C., & Smith, C. (2016). Modulation of glucocorticoid, mineralocorticoid and androgen production in H295 cells by Trimesemine™, a mesembrine-rich Sceletium extract. Journal of Ethnopharmacology, 177, 35-45.
- Tamura, Y., Nishikawa, T., Yamada, K., Yamamoto, M., & Kumagai, A. (1979). Effects of glycyrrhetinic acid and its derivatives on delta 4-5 alpha- and 5 beta-reductase in rat liver. Arzneimittel-Forschung, 29(4), 647-9.
- Taxvig, C., Elleby, A., Sonne-Hansen, K., Bonefeld-Jørgensen, E.C., Vinggaard, A.M., Lykkesfeldt, A.E., & Nellemann, C. (2010). Effects of nutrition relevant mixtures of phytoestrogens on steroidogenesis, aromatase, estrogen, and androgen activity. Nutrition and Cancer, 62(1), 122-31.
- Tominaga, Y., Nakagawa, K., Mae, T., Kitano, M., Yokota, S., Arai, T., … Inoue, S. Licorice flavonoid oil reduces total body fat and visceral fat in overweight subjects: A randomized, double-blind, placebo-controlled study. Obesity Research & Clinical Practice, 3(3), I-IV.
- Tremblay, A., Waterman, M. R., Parker, K. L., & Lehoux, J. G. (1991). Regulation of rat adrenal messenger RNA and protein levels for cytochrome P-450s and adrenodoxin by dietary sodium depletion or potassium intake. The Journal of Biological Chemistry,266(4), 2245-2251.
- Velarde, M.C. (2014). Mitochondrial and sex steroid hormone crosstalk during aging. Longevity Healthspan, 3, 2.
- Venkataramanan, R., Ramachandran, V., Komoroski, B. J., Zhang, S., Schiff, P. L., & Strom, S. C. (2000). Milk thistle, a herbal supplement, decreases the activity of CYP3A4 and uridine diphosphoglucuronosyl transferase in human hepatocyte cultures. Drug Metabolism and Disposition,28(11), 1270-1273.
- Voogt, P.A., den Besten, P.J., Kusters, G.C., & Messing, M.W. (1987). Effects of cadmium and zinc on steroid metabolism and steroid level in the sea star Asterias rubens L. Comparative Biochemistry & Physiology, 86(1), 83-9.
- Wallace, M.B., Lim, J., Cutler, A., & Bucci, L. (1999). Effects of dehydroepiandrosterone vs androstenedione supplementation in men. Medicine & Science in Sports & Exercise, 31(12), 1788-92.
- Wang, F., Zhang, Q., Zhang, X., Luo, S., Ye, D., Guo, Y., . . . Huang, Y. (2014). Preeclampsia induced by cadmium in rats is related to abnormal local glucocorticoid synthesis in placenta. Reproductive Biology and Endocrinology,12(1), 77.
- Wang, X., Wang, G., Li, X., Liu, J., Hong, T., Zhu, Q., . . . Ge, R. (2016). Suppression of rat and human androgen biosynthetic enzymes by apigenin: Possible use for the treatment of prostate cancer. Fitoterapia,111, 66-72.
- Watanabe, M., & Nakajin, S. (2004). Forskolin up-regulates aromatase (CYP19) activity and gene transcripts in the human adrenocortical carcinoma cell line H295R. Journal of Endocrinology, 180(1), 125-33.
- Weisser, H., Tunn, S., Behnke, B., & Krieg, M. (1996). Effects of the sabal serrulata extract IDS 89 and its subfractions on 5 alpha-reductase activity in human benign prostatic hyperplasia. Prostate, 28(5), 300-6.
- Weng, J.H., & Chung, B.C. (2016). Nongenomic actions of neurosteroid pregnenolone and its metabolites. Steroids, 111, 54-59.
- Wessagowit, V., Tangjaturonrusamee, C., Kootiratrakarn, T., Bunnag, T., Pimonrat, T., Muangdang, N., & Pichai, P. (2016). Treatment of male androgenetic alopecia with topical products containing Serenoa repens extract. Australasian Journal of Dermatology, 57(3), e76-82.
- Wiser, A., Gonen, O., Ghetler, Y., Shavit, T., Berkovitz, A., & Shulman, A. (2010). Addition of dehydroepiandrosterone (DHEA) for poor-responder patients before and during IVF treatment improves the pregnancy rate: a randomized prospective study. Human Reproduction, 25(10), 2496-500.
- Wojtal, K., Trojnar, M.K., & Czuczwar, S.J. (2006). Endogenous neuroprotective factors: neurosteroids. Pharmacological Reports, 58, 335-340.
- Wong, C., & Keung, W. (1999). Bovine adrenal 3β-hydroxysteroid dehydrogenase (E.C. 1.1.1.145)/5-ene-4-ene isomerase (E.C. 5.3.3.1): Characterization and its inhibition by isoflavones. The Journal of Steroid Biochemistry and Molecular Biology,71(5-6), 191-202.
- Woolveridge, I., & Peddie, M. J. (1997). The inhibition of androstenedione production in mature thecal cells from the ovary of the domestic hen (Gallus domesticus): Evidence for the involvement of progestins. Steroids,62(2), 214-220.
- Wu, X., Iguchi, T., Itoh, N., Okamoto, K., Takagi, T., Tanaka, K., & Nakanishi, T. (2008). Ascorbic acid transported by sodium-dependent vitamin C transporter 2 stimulates steroidogenesis in human choriocarcinoma cells. Endocrinology,149(1), 73-83.
- Yagci, A., Oertle, M., Seiler, H., Schmid, D., Campofranco, C., & Muller, J. (1996). Potassium induces multiple steroidogenic enzymes in cultured rat zona glomerulosa cells. Endocrinology,137(6), 2406-2414.
- Yamada, S., Akishita, M., Fukai, S., Ogawa, S., Yamaguchi, K., Matsuyama, J., … Ouchi, Y. (2010). Effects of dehydroepiandrosterone supplementation on cognitive function and activities of daily living in older women with mild to moderate cognitive impairment. Geriatrics & Gerontology International, 10(4), 280-7.
- Ye, L., Gho, W. M., Chan, F. L., Chen, S., & Leung, L. K. (2009). Dietary administration of the licorice flavonoid isoliquiritigenin deters the growth of MCF-7 cells overexpressing aromatase. International Journal of Cancer,124(5), 1028-1036.
- Zabul, P., Wozniak, M., Slominski, A., Preis, K., Gorska, M., Korozan, M., . . . Knap, N. (2015). A proposed molecular mechanism of high-dose vitamin D3 supplementation in prevention and treatment of preeclampsia. International Journal of Molecular Sciences,16(12), 13043-13064.
- Zhu, B., Loder, D. P., Cai, M. X., Ho, C. T., Huang, M. T., & Conney, A. H. (1998). Dietary administration of an extract from rosemary leaves enhances the liver microsomal metabolism of endogenous estrogens and decreases their uterotropic action in CD-1 mice. Carcinogenesis,19(10), 1821-1827.





