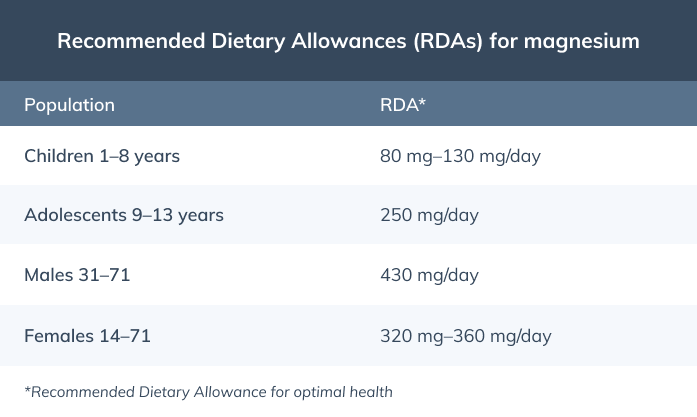
It’s best to get enough magnesium through foods; however, magnesium supplements can help you reach the Recommended Dietary Allowances (RDA) for magnesium. (3)
Did you know? Individuals with high blood pressure have a systolic blood pressure reading greater than or equal to 130 mmHg and/or a diastolic blood pressure reading greater than or equal to 80 mmHg. (1)

The FDA now allows magnesium-rich foods and magnesium supplements to bear qualified health claims stating that scientific evidence suggests that diets with adequate magnesium may reduce the risk of high blood pressure. (6)
Magnesium for lowering blood pressure: what does the research say?
A 2021 meta-analysis and systematic review concluded that magnesium supplements safely lower blood pressure in individuals with uncontrolled hypertension. Doses of at least 240 mg per day were shown to be effective in those with uncontrolled hypertension who were also taking blood pressure medication, and doses greater than 600 mg per day helped reduce blood pressure in individuals with untreated hypertension. These same benefits were not seen among individuals with controlled hypertension. Interestingly, dose mattered more than the kind of magnesium supplement. (8) A 2017 meta-analysis analyzing 11 randomized controlled trials involving participants with insulin resistance, prediabetes, or other chronic diseases, concluded that participants who received magnesium supplementation for one to six months (mean of 3.6 months) experienced a significantly greater reduction in systolic blood pressure and diastolic blood pressure compared to the control group. (2) Although there is no conclusive evidence suggesting that magnesium-rich diets or magnesium supplements can be used to treat high blood pressure, current research indicates that magnesium may be a safe, low-cost adjunctive therapy. (8)Did you know? Low dietary magnesium intake is associated with an increased risk of developing hypertension. (10)
The FDA’s stance on magnesium for high blood pressure
According to a letter issued in January 2022, the FDA now permits the use of qualified health claims on food and dietary supplement products that contain magnesium. The FDA defines qualified health claims (QHCs) as statements that are “supported by scientific evidence, but do not meet the more rigorous “significant scientific agreement” standard required for an authorized health claim.” (7) All QHCs must be reviewed by the FDA before products reach the market and must be accompanied by a disclaimer or other language that clearly explains to consumers the level of scientific evidence supporting the health claim without being misleading to consumers. (4) In their statement letter, the FDA included three approved qualified health statements that can be used by manufacturers of foods and dietary supplements that contain qualifying amounts of magnesium:- “Inconsistent and inconclusive scientific evidence suggests that diets with adequate magnesium may reduce the risk of high blood pressure (hypertension), a condition associated with many factors.”
- “Consuming diets with adequate magnesium may reduce the risk of high blood pressure (hypertension). However, the FDA has concluded that the evidence is inconsistent and inconclusive.”
- “Some scientific evidence suggests that diets with adequate magnesium may reduce the risk of high blood pressure (hypertension), a condition associated with many factors. The FDA has concluded that the scientific evidence supporting this claim is inconsistent and not conclusive.” (6)
- Disqualifying total fat levels: 13 g per label serving size
- Disqualifying saturated fat levels: 4 g per label serving size
- Disqualifying cholesterol levels: 60 mg per label serving size
- Disqualifying sodium levels: More than 480 mg per label serving size (5)
What prompted this statement by the FDA?
The FDA issued this statement following a health claim petition submitted on behalf of The Center for Magnesium Education and Research, LLC. The FDA moved forward with this decision after viewing the results of 38 intervention studies which demonstrated credible yet inconclusive scientific evidence that consumption of magnesium from foods and supplements reduced the risk of blood pressure. (5)(6)
High blood pressure (hypertension) is defined as having a systolic blood pressure reading greater than or equal to 130 mmHg or a diastolic blood pressure reading greater than or equal to 80 mmHg. (1)
The bottom line
In January 2022, the FDA stated that it will not restrict food and supplement companies from displaying qualified health claims on their products that promote the consumption of magnesium for high blood pressure. As a result, you may begin to see these health claims on food and supplement labels. While it’s best to obtain your daily magnesium needs through a healthy diet, magnesium supplements may help you reach optimal magnesium status and may provide some health benefits. If you’re a patient, always consult your integrative healthcare provider before taking new supplements.- American Heart Association. (n.d.). Understanding blood pressure readings. https://www.heart.org/en/health-topics/high-blood-pressure/understanding-blood-pressure-readings
- Dibaba, D. T., Xun, P., Song, Y., Rosanoff, A., Shechter, M., & He, K. (2017). The effect of magnesium supplementation on blood pressure in individuals with insulin resistance, prediabetes, or noncommunicable chronic diseases: A meta-analysis of randomized controlled trials. The American Journal of Clinical Nutrition, 106(3), 921–929. https://doi.org/10.3945/ajcn.117.155291
- National Institutes of Health. (2022). Magnesium. https://ods.od.nih.gov/factsheets/Magnesium-HealthProfessional/
- U.S. Food and Drug Administration. (2017). Questions and answers on health claims in food labeling. https://www.fda.gov/food/food-labeling-nutrition/questions-and-answers-health-claims-food-labeling#:~:text=An%20example%20of%20a%20qualified,of%20diabetes%20mellitus%20type%202.%E2%80%9D
- U.S. Food & Drug Administration. (2021). FDA letter in response to petition for a qualified health claim for magnesium and reduced risk of high blood pressure (docket No. FDA-2016-Q-3770). https://www.fda.gov/media/155304/download
- U.S. Food and Drug Administration. (2022). FDA announces qualified health claim for magnesium and reduced risk of high blood pressure. https://www.fda.gov/food/cfsan-constituent-updates/fda-announces-qualified-health-claim-magnesium-and-reduced-risk-high-blood-pressure
- U.S. Food and Drug Administration. (2022). Qualified health claims. https://www.fda.gov/food/food-labeling-nutrition/qualified-health-claims
- Rosanoff, A., Costello, R. B., & Johnson, G. H. (2021). Effectively prescribing oral magnesium therapy for hypertension: A categorized systematic review of 49 clinical trials. Nutrients, 13(1), 195. https://doi.org/10.3390/nu13010195
- Rosanoff, A., Weaver, C. M., & Rude, R. K. (2012). Suboptimal magnesium status in the United States: Are the health consequences underestimated?. Nutrition Reviews, 70(3), 153–164. https://doi.org/10.1111/j.1753-4887.2011.00465.x
- Schutten, J. C., Joosten, M. M., de Borst, M. H., & Bakker, S. (2018). Magnesium and blood pressure: A physiology-based approach. Advances in Chronic Kidney Disease, 25(3), 244–250. https://doi.org/10.1053/j.ackd.2017.12.003
- Serefko, A., Szopa, A., Wlaź, P., Nowak, G., Radziwoń-Zaleska, M., Skalski, M., & Poleszak, E. (2013). Magnesium in depression. Pharmacological Reports: PR, 65(3), 547–554. https://doi.org/10.1016/s1734-1140(13)71032-6





