Research Update articles are produced in order to keep practitioners up to date on impactful research that is relevant to the field of integrative medicine. These articles may contain summaries of recent studies, events, or other industry news that advance current knowledge and standards of care.
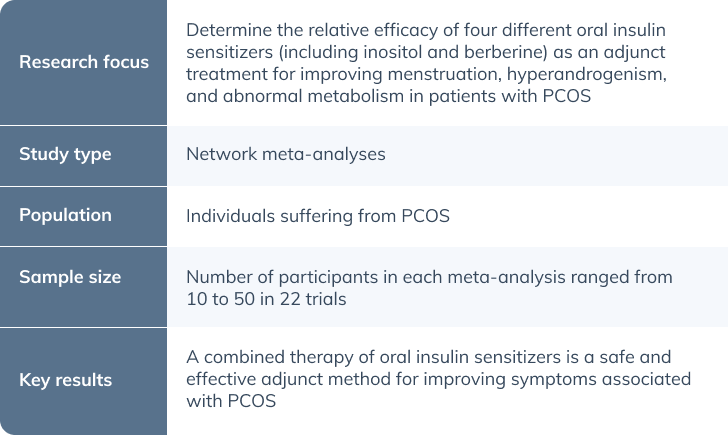
The following article summarizes the research conducted by Zhao et al. titled “Comparative efficacy of oral insulin sensitizers metformin, thiazolidinediones, inositol, and berberine in improving endocrine and metabolic profiles in women with PCOS: A network meta-analysis,” which was published in the Reproductive Health Journal. (9)
Overview
Background
Polycystic ovary syndrome (PCOS) is a highly prevalent disorder, representing the single most common endocrine-metabolic condition and affecting 4 to 21% of females of reproductive age worldwide. (5)(9) It is a complex polygenic, multifactorial disorder that currently has four recognized phenotypes, each with different health and metabolic implications:
- Hyperandrogenism and oligo-anovulation with polycystic ovarian morphology
- Hyperandrogenism and oligo-anovulation without polycystic ovarian morphology
- Hyperandrogenism and polycystic ovarian morphology but normal ovulation
- Oligo-anovulation and polycystic ovarian morphology but without hyperandrogenism (1)
Due to the complexity of the condition and the unclear mechanism of pathogenesis, the treatment strategies have remained unclear. (9) This meta-analysis aimed to alleviate this uncertainty by evaluating the effectiveness of combined therapy of four different classes of oral insulin sensitizers in PCOS compared to monotherapy. The four investigative agents were metformin (Met), thiazolidinediones (TZDs), d-chiro inositol (DCI), myo-inositol (MI), and berberine (BBR).

Method
This study gathered research to evaluate the efficacy of insulin sensitizer treatment regimens by performing traditional pairwise meta-analyses (TMA) and network meta-analyses (NMA). (9) Network meta-analysis facilitates indirect comparisons of multiple interventions that have not been studied in head-to-head studies, whereas traditional pairwise meta-analysis compares two interventions at the same time. (7)
To gather research, the authors performed systematic literature searches of studies conducted between January 2005 and October 2020 using multiple databases such as PubMed, Wanfang Data, Chinese Biomedical Literature Database (CBM), the Cochrane Central Register of Controlled Trial, among others. (9)
The search terms included different combinations of the following terms: “polycystic ovary syndrome,” “metformin,” “thiazolidinediones,” “inositol,” and “berberine.” The study used the PICO (polycystic ovary syndrome) framework to identify relevant trials. (9) More specifically, the paradigm of evidence-based medicine (EBM) recommends that physicians formulate PICO specific clinical questions and that the element of these questions would formulate a framework of knowledge around PICO. (4)
Studies included randomized controlled trials in women, aged 18 to 49 years old, that were diagnosed with PCOS based on the Rotterdam consensus, the Androgen Excess and PCOS Society criteria, the National Institute of Health, and the guidelines for diagnosis and treatment of PCOS in China. (9)
After retrieving 8,645 publications, 22 RCTs (n = 1079 participants) were included in the network meta-analysis. The chosen trials included those in which treatments were followed up for at least 12 weeks and consisted of at least one of the predefined outcomes and a comparison of the effects of insulin sensitizers. (9) Outcomes included menstruation frequency, parameters of hyperandrogenism, obesity-related indexes, parameters of glycolipid metabolism, lipid levels, and adverse effects. (9)
The excluded trials included those in which treatments combined either oral contraceptives or ovulation-inducing agents, as well as those in which patients had other diseases. (9) Furthermore, each trial was screened by two independent investigators to assess risk of bias, and discrepancies were resolved by a consensus-based discussion with another author. (9)
Results
This meta-analysis demonstrated that combination therapy with oral insulin sensitizers Met, TZDs, BBR, MI and DCI are more effective than monotherapy when treating PCOS. This was illustrated with menstrual frequency, hyperandrogenism, obesity, glycogen metabolism, and lipid levels.
In regards to improving menstrual frequency, the TMA showed that Met + TZDs was approximately four times more efficacious than Met alone, while MI + DCI was 22 times more efficacious than MI alone with low heterogeneity between studies. (9) Comparatively, the NMA revealed that MI + DCI was roughly 15 times more effective than Met as a monotherapy and 17 times more efficient than MI as a monotherapy. Overall MI + DCI was ranked best at improving menstrual frequency. (9)
The TMA and NMA both revealed that Met + BBR reduces total testosterone (TT) by an additional 11.53 ng/dL, and Met + TZDs reduces TT by an additional 3.14 to 3.66 ng/dL compared to Met alone. (9) Additionally, the NMA revealed that treatment with TZDs was less effective than Met as Met reduced TT by an average of 3.90 ng/dL more than TZDs. (9)
In regards to BMI, both the TMA and NMA demonstrated that Met + BBR reduced the BMI by nearly two times the original amount, thus demonstrating that it was superior to all other treatment options. However, they also both revealed that there was no significant difference between the groups in respect to waist-hip ratio reduction. (9)
With respect to lowering fasting plasma glucose the NMA alone revealed a difference between the investigated agents. TZDs reduced glucose by an average of 0.21 mmol/L more than Met. (9) Additionally, with regards to insulin resistance, the TMA indicated that TZDs reduced the HOMA-IR score by an additional 0.92 points, Met + TZDs reduced it by an additional 0.85 points, and Met + BBR reduced HOMA-IR by an additional 0.25 points compared to Met alone. (9)
With respect to improving blood lipids, they assessed triglycerides (TG), total cholesterol (TC), high-density lipoprotein cholesterol (HDL-C), and low-density cholesterol (LDL-C). In regards to reducing TG and TC levels, the TMA revealed that Met + TZDs reduced TGs by an additional 0.24 mmol/L and total cholesterol by 0.24 mmol/L compared to Met alone. (9) Comparatively, the NMA indicated that Met + TZDs was superior to both Met (0.08 mmol/L) and TZDs (0.51 mmol/L), and it was the best intervention among treatments for reducing levels of TG. (9) Additionally, the TMA suggested that MI was associated with higher HDL-C than Met, while NMA suggested that TZDs was superior to Met in increasing HDL-C and decreasing LDL-C. (9)
With respect to safety, TZDs had an 87 to 89% lower odds of causing an gastrointestinal adverse event compared to Met. (9) Peripheral edema was 68 times more likely to occur with TZDs as opposed to Met as measured by the NMA; no significant differences were found in the NMA. Moreover, they did not show any significant differences between the various treatment regimens for the incidence rate of muscle spasms. (9) However, these results should be interpreted with caution as they are based on a single trial. In regards to the other ingredients, such as inositol, there were no side effects described in clinical studies examining their effects.
Critical analysis
As per the authors of this review, this analysis is the first to discuss the efficacy and safety of oral insulin sensitizers as an adjunct therapy to improve irregular menses, hyperandrogenism, and glycolipid metabolism abnormalities in women with PCOS. (9)
There have been several studies on the effectiveness of individual oral insulin sensitizers. However, this is the first meta-analysis aimed at reviewing the efficacy and safety of monotherapy versus combination therapy of metformin, thiazolidinediones, myo-inositol, d-chiro inositol, and berberine. It not only incorporated direct and indirect comparisons of interventions into a single analysis, but it also provided a ranking of the available interventions. (9)
Additionally, the authors used a variety of definitions of PCOS to determine the studies that were going to be used to evaluate the different effects of these interventions. By doing so, it enabled the authors to capture more individuals affected by this condition. However, it also created the limitation associated with differentiating the efficacy of the insulin sensitizers within the various PCOS phenotypes.
Although the meta-analysis review did a comprehensive search and rigorous review of the available literature, the authors describe that there was a lack of available high-quality studies, thus creating a limited sample size of 22 trials. Not only was the literature sample size small, there was also an immense difference in the dosage, duration of treatment, and sample size within the selected studies. (9) Lastly, not all of the selected trials kept track of the women who dropped out or who missed a follow-up. (9) These limitations leave room for an increased likelihood of sample bias, selection bias, and high heterogeneity within this analysis. (9)
The bottom line
This meta-analysis is an important step in paving a pathway to remove the uncertainty around the optimal treatment approach for PCOS. The review demonstrated that myo-inositol combined with D-chiro-inositol, and metformin combined with thiazolidinediones, appear superior to metformin alone in improving insulin resistance and decreasing total testosterone. (9)
In addition, myo-inositol combined with D-chiro-inositol is particularly effective in supporting menstrual recovery. (9) Finally, thiazolidinediones and metformin combined with thiazolidinediones improve lipid metabolism better than metformin alone. (9) However, the quality and the amount of available studies was less than ideal. Therefore, this review can be used to encourage further more rigorous studies to help reduce the clinical management uncertainty for women with PCOS.
- Azziz R. (2018). Polycystic Ovary Syndrome. Obstetrics and gynecology, 132(2), 321–336.
- Azziz R, Carmina E, Dewailly D, Diamanti-Kandarakis E, Escobar-Morreale HF, Futterweit W, Janssen OE, Legro RS, Norman RJ, Taylor AE, Witchel SF, Androgen Excess Society. Positions statement: criteria for defining polycystic ovary syndrome as a predominantly hyperandrogenic syndrome: an Androgen Excess Society guideline. J Clin Endocrinol Metab. 2006;91(11):4237–45.
- Bani Mohammad, M., & Majdi Seghinsara, A. (2017). Polycystic Ovary Syndrome (PCOS), Diagnostic Criteria, and AMH. Asian Pacific journal of cancer prevention : APJCP, 18(1), 17–21.
- Huang, X., Lin, J., & Demner-Fushman, D. (2006). Evaluation of PICO as a knowledge representation for clinical questions. AMIA … Annual Symposium proceedings. AMIA Symposium, 2006, 359–363.
- Rosenfield, R. L., & Ehrmann, D. A. (2016). The Pathogenesis of Polycystic Ovary Syndrome (PCOS): The Hypothesis of PCOS as Functional Ovarian Hyperandrogenism Revisited. Endocrine reviews, 37(5), 467–520.
- Rotterdam ESHRE/ASRM-Sponsored PCOS Consensus Workshop Group. Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome (PCOS). Hum Reprod. 2004;19(1):41–7.
- Tonin, F. S., Rotta, I., Mendes, A. M., & Pontarolo, R. (2017). Network meta-analysis: a technique to gather evidence from direct and indirect comparisons. Pharmacy practice, 15(1), 943.
- Wang, F. F., Pan, J. X., Wu, Y., Zhu, Y. H., Hardiman, P. J., & Qu, F. (2018). American, European, and Chinese practice guidelines or consensuses of polycystic ovary syndrome: a comparative analysis. Journal of Zhejiang University. Science. B, 19(5), 354–363.
- Zhao, H., Xing, C., Zhang, J., & He, B. (2021). Comparative efficacy of oral insulin sensitizers metformin, thiazolidinediones, inositol, and berberine in improving endocrine and metabolic profiles in women with PCOS: a network meta-analysis. Reproductive health, 18(1), 171.


