Everything To Know About The Upcoming FDA Nutrition Facts Label Changes
As of January 1, 2020, you may notice changes to the Nutrition Facts and Supplement Facts labels found on foods and dietary supplements. These changes come as a result of the new U.S. Food and Drug Administration (FDA) requirements that will reflect updated research on nutrition and health. The new labels are designed to help individuals make better-informed, healthy dietary choices for themselves and their families. ()
This article outlines everything you need to know about the upcoming label changes.
Dietary supplement labels history
In the United States, dietary supplements are regulated by the FDA under the Dietary Supplement Health and Education Act (DSHEA). This act, passed on October 25th, 1994, defined the terms “dietary supplement” and “new dietary ingredient” (NDI). The DSHEA established a requirement for supplement manufacturers to notify the FDA of an NDI before introducing the product to the market. () The DSHEA also outlined requirements for dietary supplement labels, such as:
• The name of each ingredient
• The quantity of each ingredient
• The total quantity of a proprietary blend
• Specifications on which health claims may or may not be included (2)
The FDA announced the upcoming label changes on May 20th, 2016, which will affect both the Nutrition Facts label (found on food) and the Supplement Facts label (found on dietary supplements). ()

Supplement labels contain important information regarding the nutrients and ingredients in the product.
Upcoming label changes
With the upcoming changes, all manufacturers of food products and dietary supplements will be affected. Manufacturers with over $10 million in annual sales are required to use the new label format as of January 1st, 2020. Manufacturers with under $10 million in sales will transition to the new label by January 1st, 2021. ()
Changes to the Nutrition Facts label
The ultimate goal of these changes is to help individuals improve the quality of their diets by emphasizing the caloric content of foods, as well as identifying added sugars and nutrients that are commonly deficient. ()
Changes that you will notice with the new Nutrition Facts label are outlined below.
• The number of calories, the number of servings, and the serving size will be in larger bold font to assist with tracking calorie intake.
• Servings size requirements have been adjusted based on recent consumption data. Additionally, for packages containing more than one serving, the calories and nutrients are listed per serving and per package. (6)
• To help monitor your intake of added sugars, the amount of added sugars and percent of calories from added sugars will be listed. While the 2015-2020 Dietary Guidelines for Americans (4) recommend that added sugars account for less than ten percent of total calories consumed per day, further limiting added sugars may have additional benefits, such as a reduced risk of dental caries (cavities). (8)
• Vitamin D and potassium content will be required on the label. Meanwhile, vitamins A and C will no longer be required but may be listed voluntarily. These changes were made to reflect commonnutrient deficienciescurrently seen in the general population.
• The number of calories from fats will be removed on the new label. This change was intended to emphasize the importance of the type of fat as opposed to the total amount. For instance, monounsaturated and polyunsaturated fats may provide health benefits by reducing the risk of cardiovascular disease.
• The daily values of listed nutrients, including dietaryfiber, sodium, andvitamin D, will be updated. These new amounts will be used to calculate the % Daily Value. (6)
View the FDA’s fact sheet on the “New and Improved Nutrition Facts Label”.
The FDA label changes rely on updated scientific evidence of the associations between health and disease.
Changes to the Supplement Facts label
Similar to the Nutrition Facts label, the new FDA regulations will result in changes to the dietary supplement labels, including:
• The measurement units for vitamins A and D will change from international units (IU) to micrograms (mcg)
• The measurement unit for vitamin E will change from IU to milligrams (mg)
• As folate and folic acid are no longer considered equivalent, they will be reported in Dietary Folate Equivalents (DFE) with micrograms of folic acid in parentheses
• Niacin will be reported as milligrams Niacin Equivalents (mg NE) (3)
• As with the Nutrition Facts label, vitamins A, C, and calories from fat will no longer be required. Instead, vitamin D, potassium, and added sugars will be required.
• The daily values of nutrients will be updated to reflect current scientific evidence (5)
The changes announced in 2016 also include a definition of “dietary fiber”. The Nutrition and Supplement Facts labels may include both naturally-occurring fibers, found intact in plants, and certain added, isolated or synthetic non-digestible carbohydrates (dietary fiber). ()
The FDA currently recognizes the following ingredients as non-digestible carbohydrates:
• Beta-glucan soluble fiber
• Cellulose
• Guar gum
• Hydroxypropylmethylcellulose
• Locust bean gum
• Pectin
• Psyllium husk (1)
The new Nutrition Facts label
The image below indicates the differences between the original and new FDA Nutrition Facts labels.
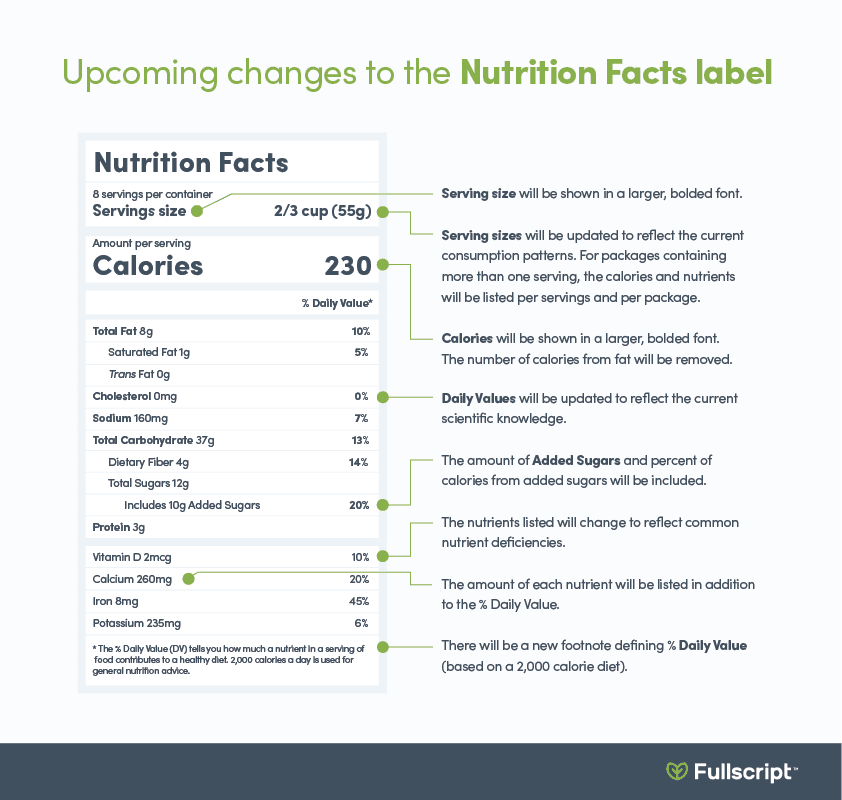
The above label changes will be required between January 1, 2020 and January 1, 2021, depending on the brand.
For information on how to read a Supplement Facts label, go to the Fullscript.
The bottom line
Understanding the upcoming changes to the Nutrition Facts labels on food and the Supplement Facts label on dietary supplements can help you to make informed choices for your health. Keep in mind, you may see both the old and new Nutrition Facts label on food products or dietary supplements during the transition period until January 1st, 2021, after which all labels will be updated. () For further information regarding dietary supplements, labeling, safety, and more visit the FDA's "".
Simplify the delivery of whole person care
• Center for Food Safety and Applied Nutrition. (2018). Questions and answers on dietary fiber. Retrieved fromhttps://www.fda.gov/food/food-labeling-nutrition/questions-and-answers-dietary-fiber
• National Institutes of Health. (1994).Dietary Supplement Health and Education Act of 1994.
• National Institutes of Health. (2019). Unit Conversions. Retrieved fromhttps://dietarysupplementdatabase.usda.nih.gov/
• U.S. Department of Health and Human Services and U.S. Department of Agriculture. (2015).2015–2020 Dietary Guidelines for Americans. 8th Edition. Retrieved fromhttp://health.gov/dietaryguidelines/2015/guidelines/
• U. S. Food and Drug Administration. (2016). Dietary supplements: New dietary ingredient notifications and related issues: Guidance for industry. Retrieved fromhttps://www.fda.gov/media/99538/download
• U. S. Food and Drug Administration. (2023). Nutrition facts label. Retrieved from https://www.fda.gov/food/nutrition-education-resources-materials/nutrition-facts-label
• U. S. Food and Drug Administration. (2019). Nutrition education resources & materials. Retrieved fromhttps://www.fda.gov/food/food-labeling-nutrition/nutrition-education-resources-materials
• World Health Organization. (2015). Guideline: Sugars intake for adults and children. Retrieved fromhttps://apps.who.int/iris/